Why Salt Melts Ice: A Molecular Gang War on Your Driveway
How does rock salt actually melt ice? It isn't heat. It is a molecular invasion called 'freezing point depression' where salt ions block water from bonding.

Quick Summary
Why Salt Melts Ice: A Molecular Gang War on Your Driveway
It is a winter ritual. The forecast calls for snow, and trucks hit the highway to spray millions of tons of rock salt. You sprinkle it on your driveway, and like magic, the ice turns to slush.
But salt isn’t hot. In fact, if you touch it, it is the same temperature as the air. So how does a cold rock melt cold ice?
The answer isn’t about heat; it is about disruption. Salt doesn’t melt ice by warming it up; it melts ice by interfering with its ability to be solid. It is a process chemists call “freezing point depression,” but you can think of it as a molecular gang war.
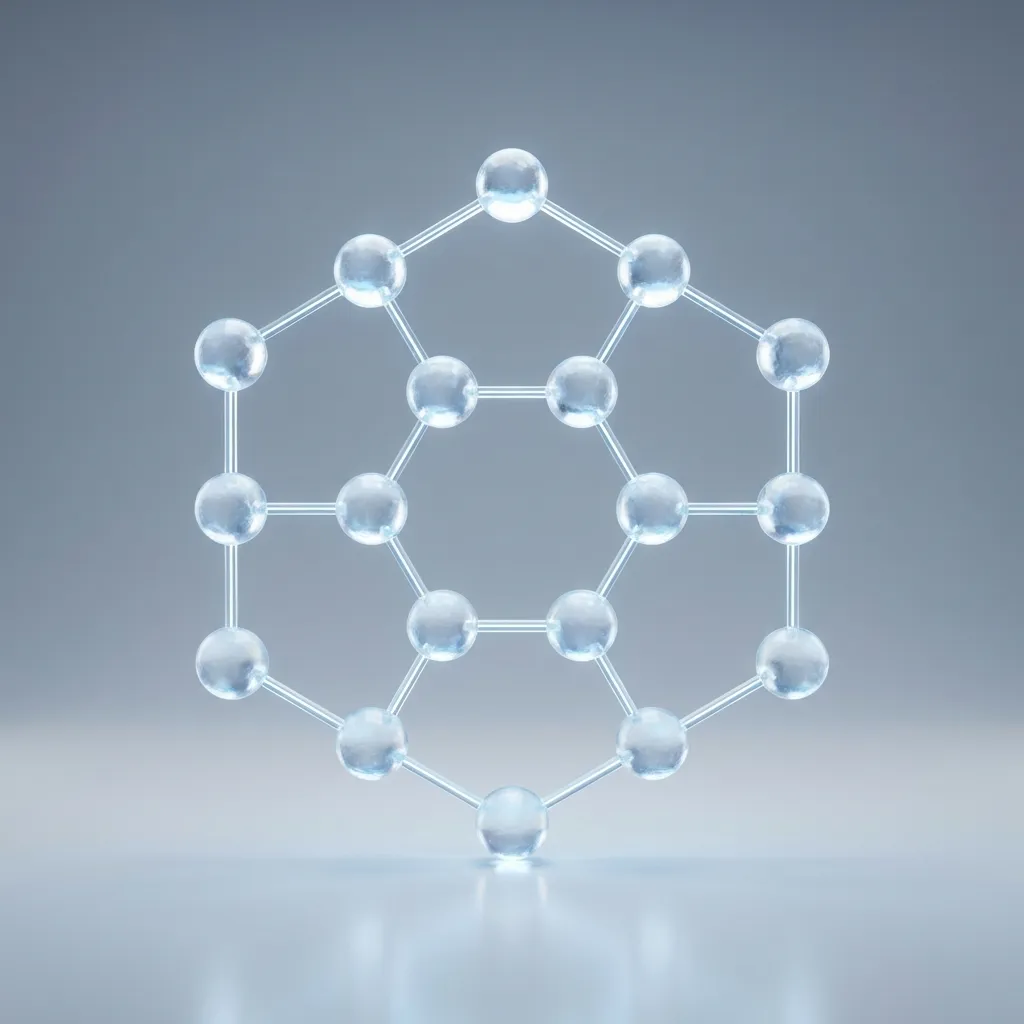
The Crystal Lattice (Ice is an Organized Club)
Ice is simply water molecules arranged in a rigid, hexagonal crystalline structure held together by hydrogen bonds. To freeze, water molecules must line up perfectly and hold hands; if they can’t organize, they stay liquid.
To understand melting, you first have to understand freezing. Water (H2O) is messy when it is liquid. The molecules tumble over each other chaotically.
But when water gets cold (32°F / 0°C), the molecules stop moving so much. They lock arms in a very specific, six-sided pattern called a crystal lattice. This structure is strict. Each water molecule must find its neighbors and form a stable hydrogen bond. If they can’t form this structure, they cannot turn into ice.
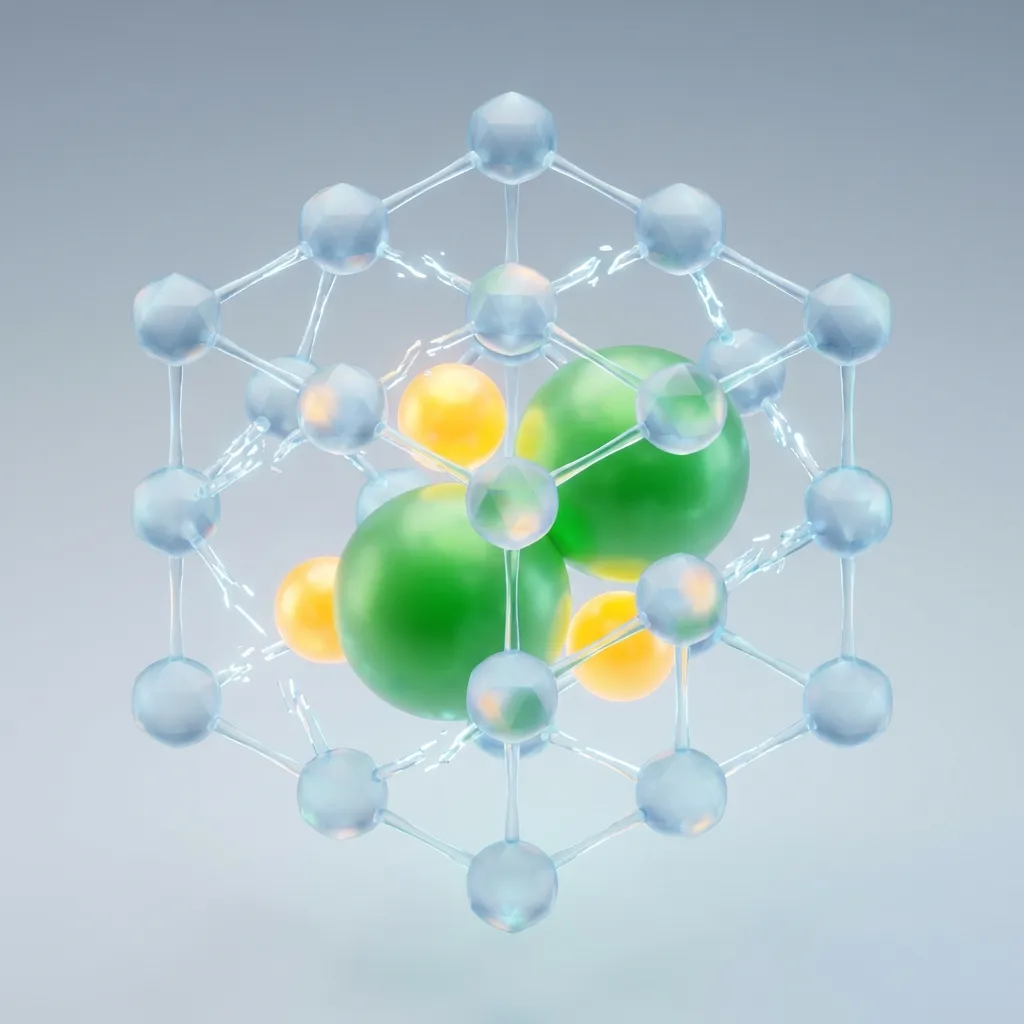
The Salt Invasion (Freezing Point Depression)
Dissolved salt ions (sodium and chloride) physically wedge themselves between water molecules, preventing them from bonding into ice crystals. This interference lowers the freezing point of water.
Enter salt (NaCl). When you throw salt on ice, it dissolves into the thin layer of surface water that’s always present. As it dissolves, it splits into two separate ions: Sodium (Na+) and Chloride (Cl-).
These ions are uniquely suited to be disruptors. They are attracted to water molecules, and they aggressively squeeze their way into the crowd.
Instead of water molecules holding hands with other water molecules to form ice, they now have these salt ions getting in the way. The salt acts like a physical blockade. The water tries to freeze, but the salt ions prevent the lattice from closing.
Because the water can’t form its crystal structure at 32°F, it stays liquid. This is “freezing point depression.” The salt has effectively changed the rules of physics for that puddle, forcing it to remain a liquid slush even when the air is below freezing.
The “Gang War” Analogy
Think of water molecules as dancers trying to hold hands to form a circle (ice). Salt ions are like rowdy intruders who run between them, breaking their grip. The more intruders you have, the harder it is for the dancers to maintain their formation.
Imagine a ballroom dance floor where people are trying to hold hands in circles. That is ice.
Now imagine someone releases a hundred energetic bulldogs (salt ions) onto the floor. The bulldogs run between the dancers, barking and jumping. The dancers can’t hold hands anymore because there is constantly a bulldog in the way.
The only way the dancers could hold hands in that chaos is if they held on tighter—which, in molecular terms, means getting even colder. But at standard freezing temperatures, the “bulldogs” win, and the circle breaks. The solid floor becomes a chaotic, liquid mess.
The 15°F Failure Point (When Salt Gives Up)
Standard rock salt stops working below 15°F (-9°C) because it needs a tiny bit of liquid water to dissolve and start the reaction. In extreme cold, standard salt is useless.
You might have noticed that when it gets incredibly cold (below 15°F or -9°C), the roads stay icy even if trucks spray them.
This is because the reaction needs a kickstart. Salt is a solid. It needs to dissolve into liquid to release those disrupting ions. Usually, the friction of tires or the sun provides just enough heat to melt a tiny layer of water on top of the ice, allowing the salt to start its work.
But in deep freezes, there is no liquid water available. The ice is too hard and dry. The salt crystals just bounce off the surface like harmless gravel. That is why in extreme climates, cities switch to calcium chloride, a different chemical that generates its own heat when it touches water, melting its way down even in sub-zero temperatures.

Tags:
About the Author
Written by Priya Sharma